
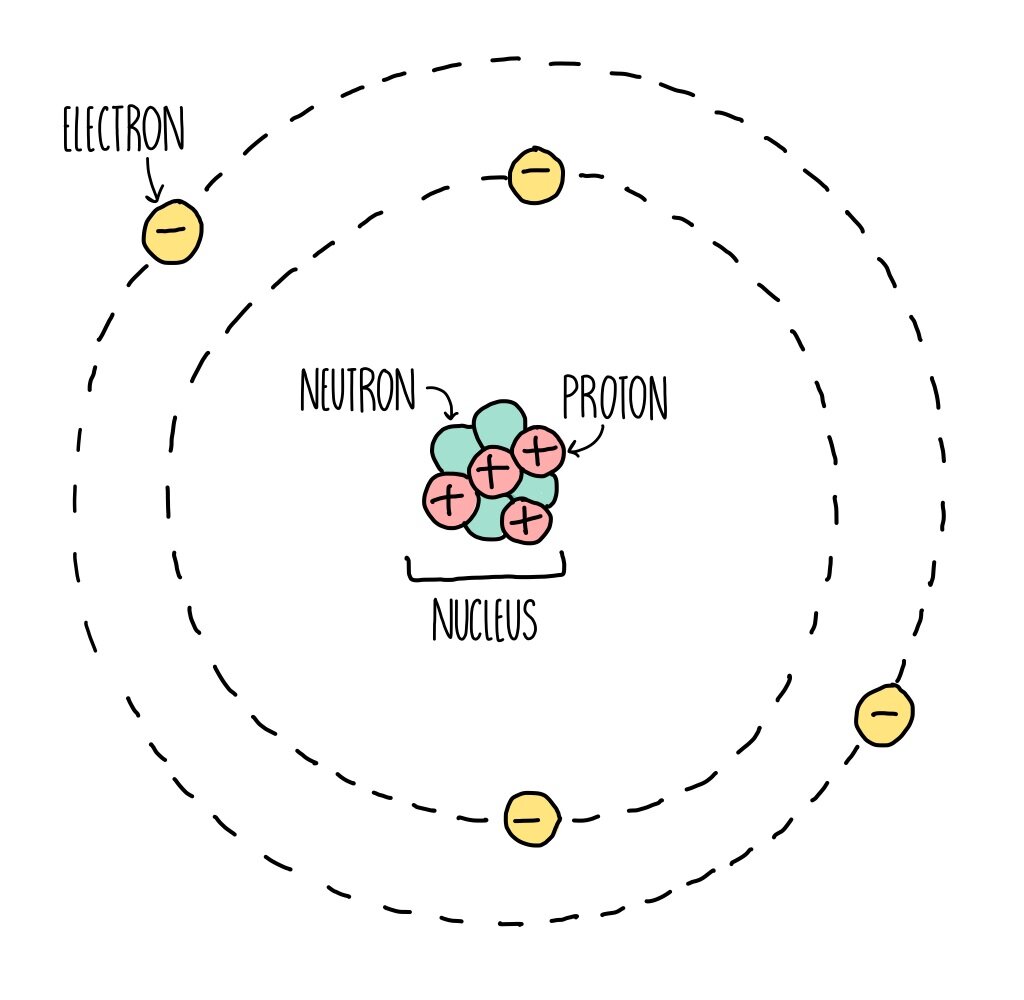
However, until the name is recognized by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of the new element is based on the Latin word(s) for its atomic number. Traditionally, the discoverer (or discoverers) of a new element names the element. All known elements and their symbols are in the periodic table. For example, Co is the symbol for the element cobalt, but CO is the notation for the compound carbon monoxide, which contains atoms of the elements carbon (C) and oxygen (O). To avoid confusion with other notations, only the first letter of a symbol is capitalized. Most symbols have one or two letters, but three-letter symbols have been used to describe some elements that have atomic numbers greater than 112. The symbols for several common elements and their atoms are listed in Table 2. Some symbols are derived from the common name of the element others are abbreviations of the name in another language. We use the same symbol to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain). For example, the symbol for mercury is Hg (Figure 3). The symbol Hg represents the element mercury regardless of the amount it could represent one atom of mercury or a large amount of mercury.Ī chemical symbol is an abbreviation that we use to indicate an element or an atom of an element. A neutral oxygen atom (Z = 8) has eight electrons, and if it gains two electrons it will become an anion with a 2− charge (8 − 10 = 2−).įigure 3. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). For example, a neutral sodium atom (Z = 11) has 11 electrons. Positively charged atoms called cations are formed when an atom loses one or more electrons. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. The charge of an atom is defined as follows:Ītomic charge = number of protons − number of electronsĪs will be discussed in more detail later, atoms (and molecules) typically acquire charge by gaining or losing electrons. When the numbers of these subatomic particles are not equal, the atom is electrically charged and is called an ion. (This isotope is known as “carbon-12” as will be discussed later in this module.) Thus, one amu is exactly \dfracĪtoms are electrically neutral if they contain the same number of positively charged protons and negatively charged electrons. Since 1961, it has been defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. The amu was originally defined based on hydrogen, the lightest element, then later in terms of oxygen. When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e). For example, a carbon atom weighs less than 2 × 10 −23 g, and an electron has a charge of less than 2 × 10 −19 C (coulomb). (credit middle: modification of work by “babyknight”/Wikimedia Commons credit right: modification of work by Paxson Woelber)Ītoms-and the protons, neutrons, and electrons that compose them-are extremely small. If an atom could be expanded to the size of a football stadium, the nucleus would be the size of a single blueberry. For a perspective about their relative sizes, consider this: If the nucleus were the size of a blueberry, the atom would be about the size of a football stadium (Figure 1).įigure 1. The diameter of an atom is on the order of 10 −10 m, whereas the diameter of the nucleus is roughly 10 −15 m-about 100,000 times smaller. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The development of modern atomic theory revealed much about the inner structure of atoms.

Define the atomic mass unit and average atomic mass.Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion.


 0 kommentar(er)
0 kommentar(er)
